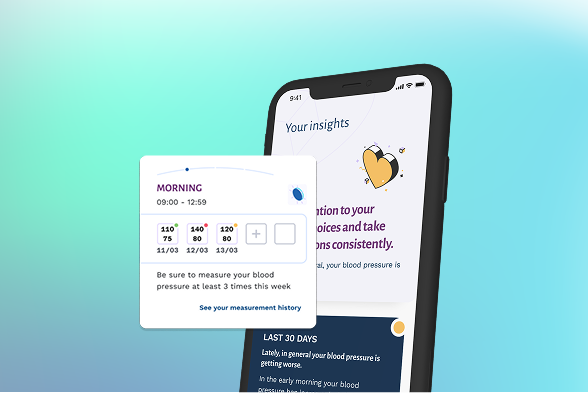
Amicomed
Cardiometabolic Disease
Newel Health co-develops evidence-based therapeutics, digital biomarkers, endpoints, and companion apps with pharma and medtech.
We develop digital medical devices to support patients with conditions across the cardiometabolic spectrum, including hypertension, heart failure, and obesity, where sustained lifestyle change and daily disease management are critical to health outcomes.
Our solutions in Parkinson’s disease and other neurological conditions help patients and clinicians manage complex, progressive symptoms through continuous monitoring and personalized support.
Chronic pain requires more than episodic treatment. Our evidence-based digital medical devices deliver scalable behavioral interventions that improve daily function and quality of life.
As a legal manufacturer of software as a medical device (SaMD), Newel Health ensures every product meets the highest regulatory standards (ISO 13485, MDR, FDA-aligned). We take on the full compliance responsibility, from design control to post-market surveillance, so partners can focus on therapeutic innovation. This makes us a trusted route for pharmaceutical companies and healthcare systems to bring digital therapeutics to market safely, credibly, and at scale.
H.Core is the engine behind Newel Health’s digital medical devices, integrating regulatory compliance, behavioral science, AI, and real-world data into one powerful platform. It enables us and our pharmaceutical partners to rapidly build, test, and scale clinical-grade software that is safe, patient-centric, and regulator-approved. From digital endpoints and remote monitoring to patient support programs and decision support, H.Core turns data and design into medical-grade solutions that sustain outcomes in trials and beyond.
Our pipeline demonstrates the strength of Newel Health’s execution capabilities, spanning cardiometabolic disease, Parkinson’s disease, and chronic pain. With CE-marked products already in market and additional assets advancing through development, we combine regulatory expertise, behavioral science, and clinical rigor to deliver digital medical devices that are both medically credible and scalable. This breadth of therapeutic focus underscores our ability to partner with pharmaceutical companies and healthcare systems to bring digital care to patients worldwide.
CE-marked and regulator-approved solutions that pharma partners can brand, deploy, and scale with confidence.

Cardiometabolic Disease

Chronic Musculoskeletal Pain

Parkinson’s Disease
Our recent news and insights highlight the latest developments, achievements, and thought leadership shaping our journey forward. From product innovations and strategic partnerships to industry trends

Newel Health announces that ROHKEA® VR Therapy has received MDR Class IIa

When the first wave of digital therapeutics (DTx) entered the market, the mission was clear: demonstrate clinical efficacy through...

We are delighted to announce a significant accomplishment at Newel Health – the attainment of not just one, but three prestigious international certifications: ISO27001, ISO13485, and ISO9001.